This is in stark contrast to chlorine which has been extensively documented to form trihalomethanes thms such as chloroform and halogenated organic compounds.
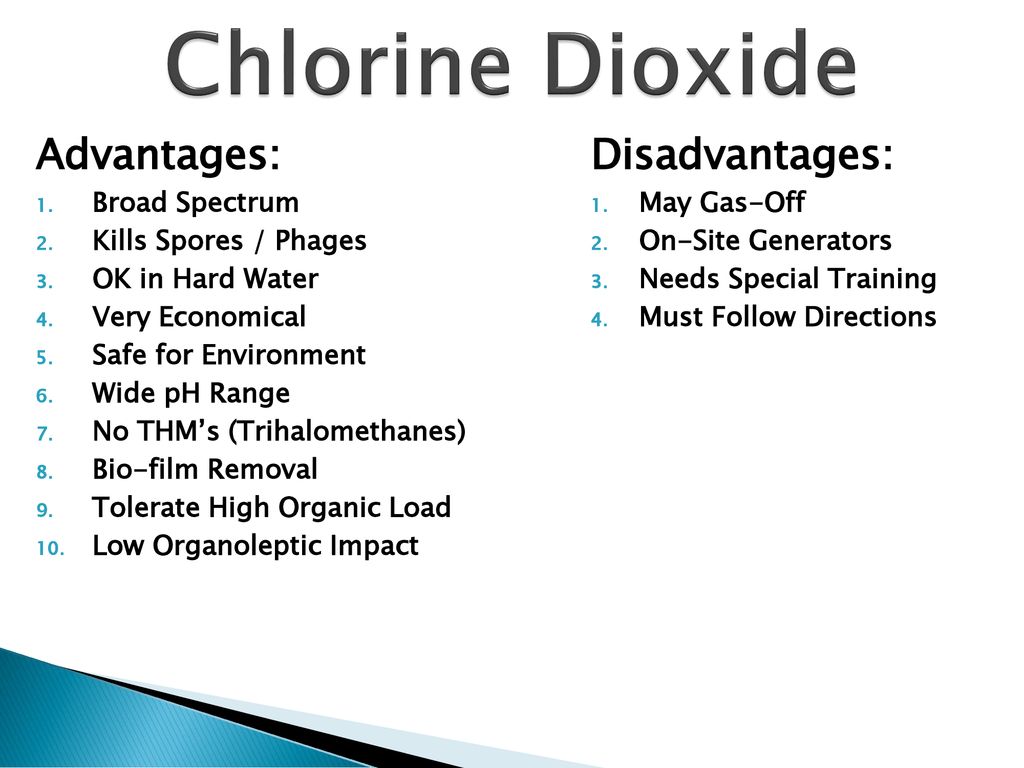
Chlorine dioxide water treatment disadvantages.
For drinking water treatment chlorine dioxide can be used both as a disinfectant and as an oxidizing agent.
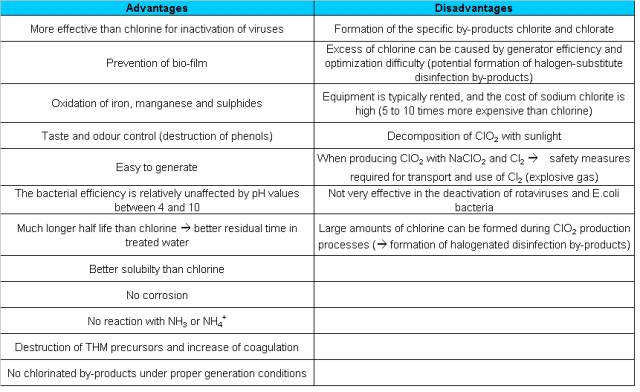
These are harmless by products.
Chlorine dioxide on the other hand provides disadvantages that outweigh the advantages.
Overview information chlorine dioxide is a gas.
These precursors meet the strictest quality and compliance standards in the usa and canada including awwa b303 19 nsf 60 and en 938 quality standards.
The most important one is the fact that it is simply not an effective water treatment to completely eradicate biofilm.
Chlorine dioxide ends up releasing oxygen and forming nacl salt in water.
However it is generally not considered a competitive technology for wastewater disinfection since it offers no significant technological advantage compared to chlorine because additional salts i e sodium thiosulfate.
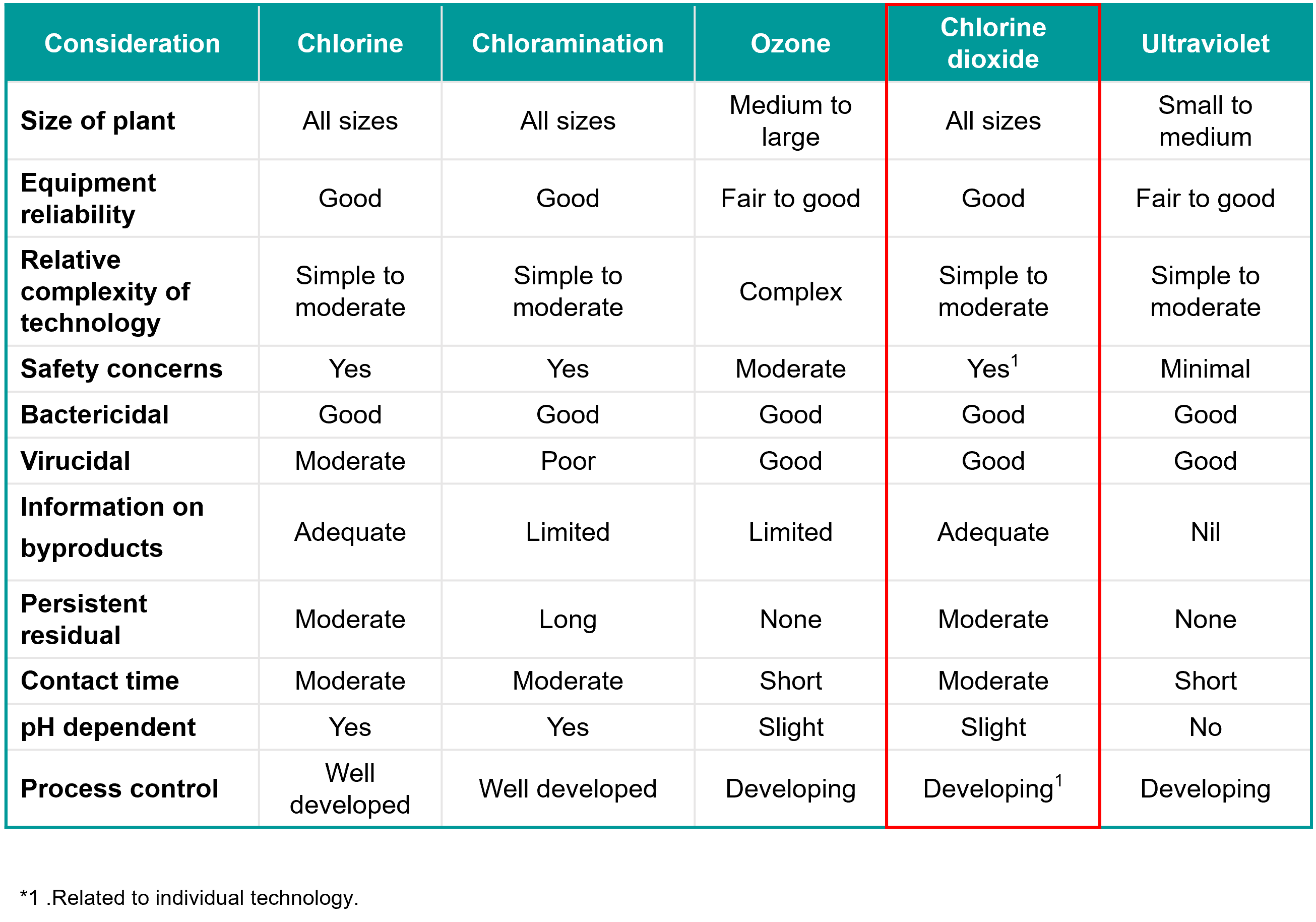
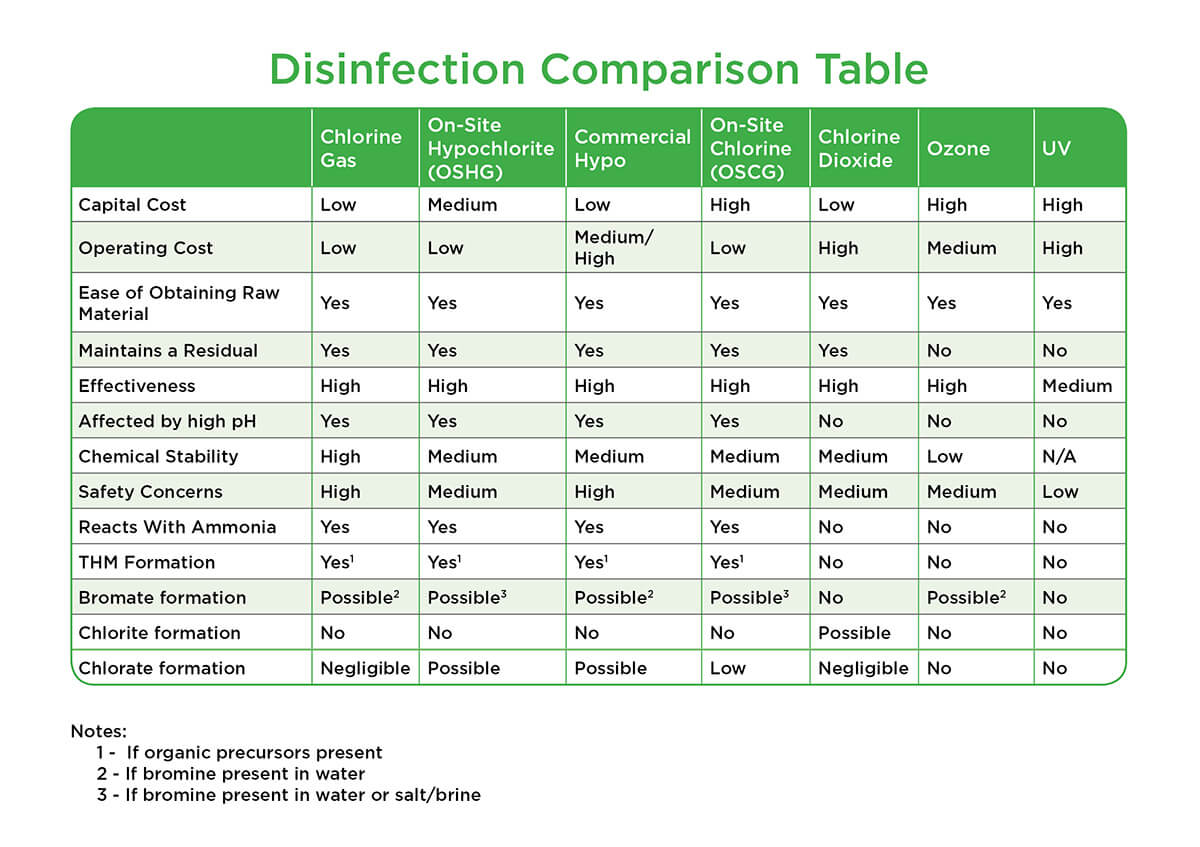
Chloramines chlorine dioxide clo2 ozone o3 ultraviolet radiation uv what are some of their advantages and disadvantages.
These alternate disinfectants for drinking water treatment include.
Chlorine dioxide is currently used in about 13 of the drinking water treatment facilities in the us usepa draft 1998.
The following table shows these advantages.
Chlorine dioxide is currently used in about 13 of the drinking water treatment facilities in the us usepa draft 1998.
By adding chlorine dioxide in the pre oxidation stage of surface water treatment the growth of algae and bacteria can be prevented in the following stages.
Chlorine dioxide clo 2 is a versatile broad spectrum biocide with a 75 year track record of safe and effective drinking water treatment industrial water treatment and wastewater treatment it selectively oxidizes biological pathogens without co generating trihalomethanes thms bromates and other toxic disinfection by products dbps.
Chlorine dioxide is generated on site using either precursor sodium chlorite or sodium chlorate.
It is commonly used to disinfect drinking water.
It can be used for both pre oxidation and post oxidation steps.
It is an oxidizing agent able to transfer oxygen to a variety of substrates while gaining one or more electrons via oxidation reduction it does not hydrolyze when it enters water and.
May choose to use alternate disinfectants.
When used in very small quantities to disinfect water it is safe and does not lead to health risks.